Faculty Member Contributes to Groundbreaking Research
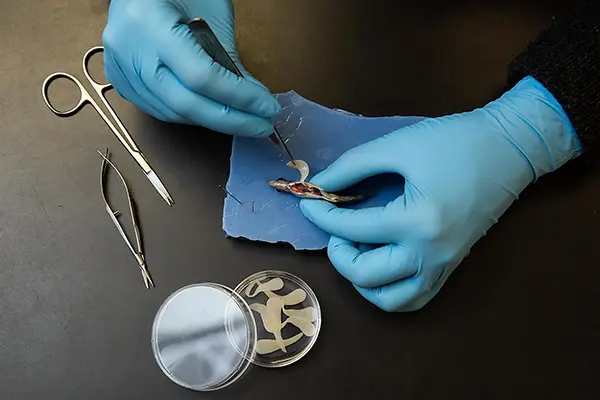 Image by Jeremy Salapek
Image by Jeremy Salapek
09/13/2022
Media Contacts: Emily Gowdey-Backus, Emily_GowdeyBackus@uml.edu and Nancy Cicco, Nancy_Cicco@uml.edu
Lugging around a tapeworm that’s one-third your body weight can be a real drag. So threespine stickleback fish evolved resistance to tapeworms — but resistance has costs of its own, a team of researchers show in an article published in Science Thursday.
When threespine stickleback fish left marine waters to colonize northern freshwater lakes roughly 12,000 years ago, they encountered freshwater tapeworms. The parasites invaded their abdomens and grew, reaching enormous sizes up to one-third of the host fish’s body weight. That's the equivalent of a human carrying a 50-pound tapeworm. However, some stickleback populations quickly evolved a defense. Upon encountering a tapeworm, their immune systems would form scar tissue around the parasite, stopping its growth. But other stickleback populations instead tolerated the worms, scarring only a little or not at all.
Groups of sticklebacks that scar against tapeworms, and those that don’t, can live quite close to each other in lakes just miles apart. Until now, no one has understood why some stickleback populations evolved one way and some another.
“This work is important as it highlights the immunologic variability (and therefore the ability to resist infections) that exists within and between populations, how it comes about, and how it can affect health outcomes,” said Natalie Steinel, an assistant professor of biology at the University of Massachusetts Lowell and associate director of the university’s Center for Pathogen Research & Training.
“We see this in Alaska, in British Columbia. Colleagues have seen it in Scandinavia,” says University of Connecticut biologist Dan Bolnick.
“The neat thing about coevolution between tapeworms and fish is that it’s a remarkably dynamic process, and there are different outcomes to this evolutionary battle in every place that we look,” says Jesse Weber, a biologist at the University of Wisconsin Madison.
Bolnick, Weber and Steinel worked together to answer the question of stickleback parasite resistance. Along the way, they showed resistance to parasites isn’t always a good thing.
The trio studied sticklebacks from Roberts and Gosling lakes on Vancouver Island in British Columbia. Both lakes have tapeworms and both have sticklebacks. The two populations of stickleback fish are extremely similar. The major difference is that Roberts fish scar aggressively to prevent tapeworms from growing while Gosling fish do not. The only other obvious difference is that Roberts females reproduce much less successfully than Gosling females, apparently because the scar tissue in their abdomens makes it more difficult.
The researchers wanted to know which genes were responsible for the scarring and whether the scarring was the reason Roberts females didn’t reproduce as well. But if they simply compared the genomes of Roberts and Gosling fish directly, they might be confused by other genetic differences between the populations that were irrelevant to scarring. They had to mix the two populations so the only consistent difference between the two was the scarring trait.
To reshuffle the genetic deck, the researchers crossbred fish from Roberts and Gosling. These Roberts-Gosling hybrids were all similar, each having half their genes from each population. Then these hybrids were mated to create a second generation. The second generation had many different combinations of genes with individual fish having varied traits from each other, their hybrid parents, and from the Roberts-Gosling grandparent generation.
It was this second, genetically shuffled generation that researchers exposed to tapeworms.
After exposing them for a specific number of days, the team looked at the relative amounts of scarring and tapeworms in each fish. They analyzed the genomes of fish with a heavy worm load, comparing it with the DNA of fish with heavy scarring. They narrowed the differences down to a handful of genes and looked carefully to see which of the genes was very active.
They found one of the most active genes was one that is also closely associated with scarring in mice.
You might be surprised mice scar in the same way as fish. But scarring is controlled by the immune system, which is similar in all vertebrates, from fish to mice to humans.
The researchers then looked at that gene in the two original populations. In the genome of the Gosling sticklebacks, the fish that tolerate tapeworms without scarring, researchers found that the gene had recently evolved. There seemed to be constant evolutionary pressure to tolerate tapeworms instead of scarring them in.
UMass Lowell’s Steinel said: “In this article, we’re addressing the question of immune/pathogen co-evolution using fish, but these principles are broadly applicable to other animal systems, including human infections. To successfully manage infectious diseases, we must understand the balance of costs and benefits that results from an immune response.”
“This is one of a very few papers done in both the wild and the lab to show a big fitness cost” to parasite resistance, Bolnick said. But it makes sense. Female sticklebacks with lots of scarring are 80% less likely to successfully breed. Tapeworms don’t seem to affect breeding, although they do slow the fish down and make it more likely to be eaten by a bird.
“As we jump around and look at these systems, we can learn an awful lot not just about the process of evolution, but also about new mechanisms with applied value to people and livestock – mechanisms such as how your immune system recognizes a parasite, how you resist a parasite, and how you turn off an unwanted immune response,” Weber said.
This work was funded by the Howard Hughes Medical Institute Early Career Scientist fellowship, as well as grants from the National Institutes of Health.
